Battery Microcalorimetry
When a Li-ion battery is charged or discharged, heat is generated from the electrodes and internal reactions in the cell1.Őż The TAM III isothermal calorimeter (TA Instruments), pictured below, measures the heat flow of a cell with an accuracy better than ¬Ī 1 őľW.
This heat flow, combined with the simultaneously collected electrochemical data, can be used for a wide variety of applications, including:
‚ÄĘ Quantifying the energy produced from unwanted parasitic reactions between electrodes and electrolytes2,3
‚ÄĘ Studying the effect of electrolyte additives on electrode-electrolyte reactivity,
‚ÄĘ Combined with theoretical calculations to characterize solid electrolyte interphase products4

Figure 1 - TAM III Microcalorimeter
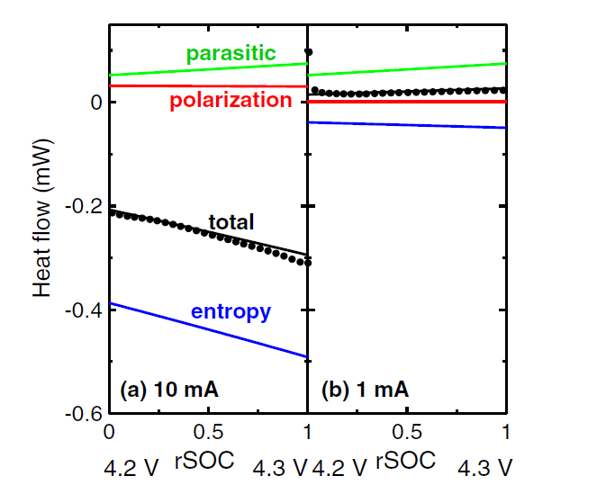
Figure 2 - Heat flow measurements can be modelled to extract the various components of the heat flow in a Li-ion cell.Őż The parasitic heat flow is the source of the eventual ‚Äúdeath‚ÄĚ of Li-ion cells. See references 1, 2 and 3 for more information.
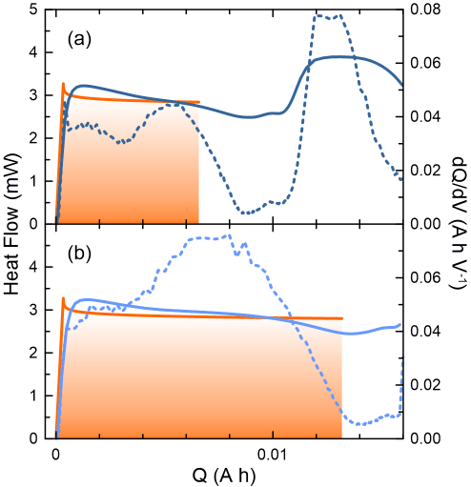
Figure 3 - During the first cycle of a 0.220 Ah pouch cell 2% (a) and 4% (b) by weight of an electrolyte additive is reduced on the negative electrode. The measured heat flow (solid blue) is compared to a theoretical value (orange) calculated for a specific reaction scheme determined using quantum chemistry (see reference 4).
Őż
References:
- ŐżL.J. Krause, et al., J. Electrochem. Soc. 159, A937 (2012)
- ŐżDownie, L.E., et al., J. Electrochem. Soc. 161,ŐżA1782, (2014)
- ŐżS. Glazier, et al., J. Electrochem. Soc. 163, A2131, (2016)
- ŐżHall, D.S., et al., Phys. Chem. Chem. Phys. 18, 11383, (2016)