Symmetric cells
Symmetric Li-ion cells have the same material as the positive and negative electrode (when cells are assembled one would already contain lithium and the other wouldn鈥檛).听 Although the cells have an average voltage of zero volts and are useless from a practical point of view, the can give vast information about reactions between electrode materials and electrolyte.
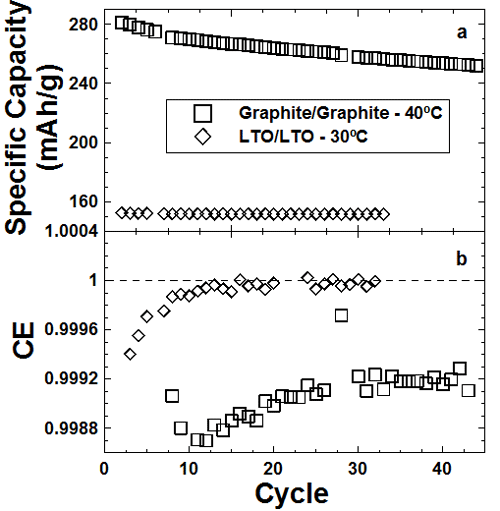
Figure 1
The top panel of the Figure shows capacity versus cycle number for graphite/graphite and Li4/3Ti5/3O4/ Li4/3Ti5/3O4 symmetric cells. The LTO cell cycles without loss and with almost perfect coulombic efficiency (bottom panel). This shows that electrode/electrolyte reactions are much slower on LTO than graphite.
听
听
References
- J.C. Burns, L.J. Krause, D.-B. Le, L.D. Jensen, A.J. Smith, D. Xiong, and J.R. Dahn. J. Electrochem. Soc. 158, A1417-A1422 (2011).
- Xin Xia, Ping Ping and J.R. Dahn, J. Electrochem. Soc. 159, A1460-A1466 (2012).
- P. Ping, Q.S. Wang, J.H. Sun, X. Xia and J.R. Dahn, J. Electrochem. Soc. 159, A1467-A1473 (2012).
听
